Chapter 3: Data Collection and Storage
Module 8: Informed Consent and Assent
Have you ever clicked “I agree” without reading the terms and conditions?
In research, consent isn’t just a formality; it’s about ensuring participants genuinely understand what they’re signing up for. This module covers what informed consent and assent mean, how to support informed decision-making, who can legally consent, and implications for documentation in digital research.
Learning Objectives
- Learn about informed consent and assent in the context of online survey research
Case Study
Linda, a research postgraduate student, is working with older adults diagnosed with Alzheimer’s dementia. One participant, Mr. Jensen, eagerly consents, but his daughter later contacts Linda, claiming he lacks the capacity to understand the study and would like his participation rescinded. Mr. Jensen insists on participating, asserting his independence. He asks Linda, “Why can’t I participate? You can’t keep me from doing this!”
🧐 Consider This!
Where do you believe the line for informed consent is? Who can consent?
What is informed ?
Informed consent by definition is to voluntarily agree to an action or procedure using all necessary information to make a rational decision. This process should be informed, given voluntarily, and be an ongoing process- but how do we do that for online surveys? Let’s dive in!
1. Consent Should Be Informed
For someone’s consent to be informed, one must be able to understand the information provided. As detailed in the Language Matters module, using simple language is vital to participant understanding. All participants should also be given ample time to consider their decision with opportunities to ask any questions before agreeing and participating.
In an online survey setting, the following can ensure that participants are well-informed:
- Letters of information and letter of consent (consent documents) are available in multiple languages, written in lay terms.
- Consent documents are screen-reader accessible, and written in an accessible font.
- Consent is culturally appropriate (consider all populations, including Indigenous communities; see: TCPS 2 (2022) Chapter 9).
- Consent information is available with relevant accessible language accommodations (See: Language Matters).
- Contact information of the research team is available to provide responses to questions, and support (including e-mail address, phone number, and/or physical address).
2. Informed Consent Should Be Given Voluntarily
Voluntary consent refers to consent that was given without coercion or undue influence, according to their own values, preferences and desires. For example, when a research study is offering disproportionally large incentives, or a recruiter is someone of authority to the potential participant, the voluntary nature of a participant’s consent may be compromised.
Furthermore, participants have the right to withdraw from the research project at any time without facing any negative consequences. This includes the option to withdraw any data they have previously provided to the researchers.
In online survey research, the way to withdraw from research should be made very clear for participants. Before a survey is completed and submitted, participation can be ended whenever a participant exits the online survey window.
However, once a survey has been completed, there may be no way to withdraw participation without being identified, as participants will need to contact the research team in order to withdraw information.
Make these conditions clear in your letter of information and survey preamble.
As always, transparency is key!
3. Consent Is Ongoing
From the moment a participant is required through every step afterwards, the participants consent must be maintained. This means that if any changes are made to the research through its duration that may change a participants willingness to continue, it must be shared and opportunity given to withdraw.
In online survey settings, participants are able to exit the survey easily at any time, by clicking out of the survey, with no repercussions. This makes ongoing consent relatively simple for online survey research.
Module 8, Activity 1
Who can consent?
Who can legally consent to participate in research depends on factors like age, mental capacity, and legal jurisdiction. That being said, ethics go beyond what is legal. Just because someone is legally permitted to consent doesn’t mean they will always fully understand the implications.
There must be accommodations made to ensure your participants are able to fully understand what they are consenting to. For example, if your research is focused on persons with dyslexia, using a dyslexic friendly font is a useful strategy.
For more information on this, you can see the TCPS2: CORE 2022 guidelines around informed consent and assent.
As a researcher, determining who can consent takes serious consideration of many factors such as, risk level, characteristics of the research participant or even the topic of the research itself.
However we must be mindful of using rigid checklists to gauge whether to include or exclude certain individuals without any formal assessments. Following a one-size-fits-all approach to assessing capacity for consent can lead to developing significant, ableist biases, which can produce non-representative results.
🧐 Consider This!
Research Directives are a proposed type of advance directive that asserts an individual’s preferences for participation in future research in the event that they lose capacity to consent.
This type of directive is not legally binding, and has not been tested yet. However, research directives align with the TCPS 2 (2022) core principle of Respect for Persons, and thus, show promise for future applications.
What do you think about this proposed form of participation?
What is informed ?
Assent is similar to informed consent, but refers to the voluntary participation of those who are unable to legally give consent, either due to age or capacity. Legal consent must still be given by a legal guardian in order for research to continue.
is the inverse of this agreement, meaning that the participant has signs suggesting they do not wish to participate. Dissent of a participant will override a guardian’s consent on behalf of the participant, and the researcher should not continue if they identify dissent.
Assent and dissent to participation may apply for individuals such as:
… Whose decision-making capacity is in the process of development and maturation;
… Who once were capable of making an autonomous decision regarding consent but whose decision-making capacity is diminishing or fluctuating; and
…Whose decision-making capacity is partially developed.
In Canada, assent can be acquired in multiple ways, depending on the evaluated or perceived capacity of each participant. Participants may be able to sign a regular consent document, or a separate assent document that is appropriate for their comprehension level, or the participants may provide verbal assent. These decisions can be made with the participant, and alongside the legal guardian of the participant, who must also consent to the participant’s involvement in the study.
Capacity to assent is often assessed before beginning a research activity, where participants are asked simple questions about the research topic, the type of activity, and their rights as participants. If a participant is deemed unable to consent, then the research activity may be discontinued.
For example:
A 9-year-old child is asked to participate in an interview with a researcher about their experience with online classes as an elementary school student. The researcher provides an age-appropriate letter of information, as well as a letter of assent, for the child to sign. The researcher also provides the child’s parent with a standard letter of information and a consent form, for them to sign as a guardian of the child. Both the child and parent sign the forms after asking and receiving answers to some questions.
However, before the interview begins, the researcher asks the child a capacity assessment question: “What are we going to talk about today?”
The child is unsure, so the researcher goes through the letter of information again, using age-appropriate language.
When the researcher asks again, the child answers: “We are going to talk about online school.” After two more correct responses to assessment questions, the researcher is confident in the child’s capacity to assent and begins the interview.
For online survey research, it is important that we are wary of those who assent to participation, as the research activity requires that participants are able to provide responses to specific questions. In the case that we are uncertain of a participant’s ability to consent, we can use screening assessment questions in order to gauge capacity.
These might resemble a multiple choice question, such as: “What is this survey about?”
What is this survey about?
a. It is about online school.
b. It is about my health.
c. It is about my family.
d. I don’t know.
If a participant is ultimately unable to consent for online survey research, even when provided simple text and disability accessible versions of consent documents, there are limited solutions that can be used in order to collect data.
One might be to have a trusted guardian or support person complete the survey on behalf of someone, from their personal perspective. This data will obviously be biased and skewed according to the perspective of the respondent– as such, including a question about the perspective of the survey participant should be included. For example: “From what perspective are you completing this survey?”
From what perspective are you completing this survey?
a. I am an elementary school student
b. I am the parent/legal guardian of an elementary school student
While this is prone to biases of the respondent, this strategy might help you collect some data in areas where it may otherwise be impossible.
Supporting Informed Decision-Making
There are many things you can do as the researcher to support your participant through the consent decision making process.
- Verbal/video-recorded explanations of consent forms/procedures.
- Using plain language at about a grade 7 reading level.
- Providing as much time as needed for participants to make a decision.
- Prioritizing digital accessibility (for example, screen-reader friendly materials).
- Using comprehension checks to verify understanding.
- Providing spaces and ways to ask questions.

Documenting Consent
Overall, consent forms can follow the same general structure:
- The type of research being performed;
- The name(s) of the investigator(s) conducting the study;
- The potential risks and benefits of the project;
- What their participation involves;
- Any potential, real, or perceived conflicts of interest;
- Any applicable funding sources;
- Any incentives, reimbursements, costs, or compensation relevant to the study;
- How confidentiality or anonymity will be maintained;
- How participants may withdraw from the study; and
- Who will have access to the data, and how the data will be managed and destroyed.
Taken from: “GUIDELINES FOR OBTAINING CONSENT AND ASSENT” (Toronto Metropolitan University, 2017)

Assent forms follow a similar format, but use simple, capacity-appropriate language.
Whether consent and assent is done digitally, verbally, or on paper depends on the study and participant preference, capabilities and security concerns.
Consult your own institution’s Research Ethics Board to find out their consent and assent form guidelines.
For online survey research, consent is typically collected using a Survey Consent Preamble. This is a survey page containing the survey letter of information and consent form, which precedes the main survey questions. The main survey should only be made accessible to participants who have consented and submitted the consent preamble.
A question to ask for a participant’s consent may resemble the following:

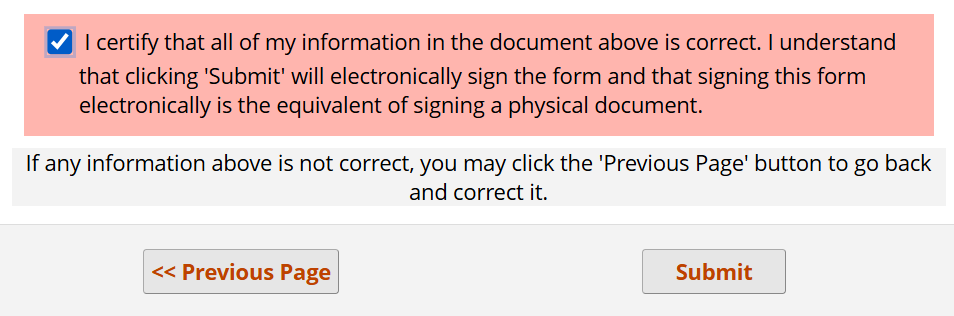
Submission of this survey consent preamble may resemble the following:
To voluntarily agree to an action or procedure using all necessary information to make a rational decision.
The voluntary participation of those who are unable to legally give consent, either due to age or capacity.
the inverse of "assent". When a person who is unable to legally consent indicates that they do not wish to participate.



Feedback/Errata