Outpatient GI
Background
Page Contents
The Gastrointestinal (GI) Tract
There are three main functions of the gastrointestinal tract, including transportation, digestion, and absorption of food. This takes place in three different phases of digestion: , , and .
The gastrointestinal system begins with the mouth, leading to the esophagus and extends through the stomach, small and large intestine to the anus. Food is digested in the stomach and nutrients are absorbed in the small intestine. In the large intestine, water is reabsorbed and fecal matter is expelled through the rectum. The pancreas, liver, and gallbladder also have a role in digestion.
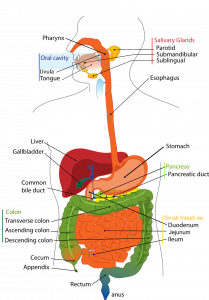
Source: Sarahguess5, CC0, via Wikimedia Commons
GI Tract Components
The table below provides a brief overview of the different GI tract components, outlining a process that starts in the oral cavity extending to the large intestine in preparation for elimination.
| Component | Brief Explanation |
|---|---|
| Oral Cavity, Teeth and Tongue | Mechanical processing, moistening, and mixing with salivary secretions. Carbohydrate digestion begins in the mouth via enzymatic action (i.e. salivary amylase). |
| Salivary Glands | Secretion of lubricating fluid containing enzymes that breakdown carbohydrates. |
| Pharynx | Pharyngeal muscles propel materials into the esophagus. |
| Esophagus | Transport of materials to the stomach. |
| Stomach | Chemical breakdown of materials via acid and enzymes; mechanical processing through muscular contractions. Protein digestion occurs in the stomach (i.e. pepsin). |
| Liver | Secretion of bile (important for lipid digestion), storage of nutrients, and many other vital functions. |
| Gallbladder | Storage and concentration of bile. |
| Pancreas | Exocrine cells secrete buffers and digestive enzymes; endocrine cells secrete hormones. |
| Small Intestine | Enzymatic digestion and absorption of water, nutrients, vitamins, and ions. Carbohydrates (amylase), fats (lipase and bile) and protein digestion further occurs in the small intestine (trypsin). |
| Large Intestine | Dehydration and compaction of indigestible materials in preparation for elimination. |
Digestive Hormones or Enzymes
The table below provides a brief overview of key digestive hormones or enzymes in the GI tract. These have been selected as a refresher of the physiological mechanisms and localizations.
| Hormone or Enzyme | Localization | Main Physiologic Actions |
|---|---|---|
| Amylase | Mouth (salivary amylase) and pancreas |
Initiates the digestion of carbohydrates in the form of starches by catalyzing the hydrolysis of polysaccharides into disaccharides.
|
| Lipase | Mouth, stomach and pancreas |
A lipase is any enzyme that catalyzes the hydrolysis of fats. Lipases perform essential roles in digestion, transport and processing of dietary lipids.
|
| Pepsin | Stomach |
Pepsin is an endopeptidase that breaks down proteins into smaller amino acids. It helps digest the proteins in food.
|
| Gastrin | Stomach, duodenum, pancreas |
Gastrin is a peptide hormone that stimulates secretion of gastric acid () by the parietal cells of the stomach and aids in gastric motility.
|
| Secretin | Small intestine (duodenum) |
Secretin is a hormone that regulates water homeostasis throughout the body and influences the environment of the duodenum by regulating secretions in the stomach, pancreas, and liver.
It helps regulate the pH of the duodenum by (1) inhibiting the secretion of gastric acid from the parietal cells of the stomach and (2) stimulating the production of bicarbonate from the ductal cells of the pancreas.
|
| Cholecystokinin (CCK) | Small intestine (duodenum) | CCK is a peptide hormone of the gastrointestinal system responsible for stimulating the digestion of fat and protein. It is released after a meal, aiding in digestion and reducing appetite. |
| Motilin | Small intestine (duodenum and jejunum) | Motilin is a hormone that helps control smooth muscle contractions in the upper gastrointestinal tract. It increases the migrating myoelectric complex, which moves food through the stomach and small intestine, and also stimulates the production of pepsin. |
| Gastric inhibitory peptide (GIP) | Small intestine (duodenum) |
Secreted from the intestine on ingestion of glucose or nutrients to stimulate insulin secretion from pancreatic β cells.
|
| Glucagon-like peptide-1 (GLP-1) | Small intestine (distal ileum and colon) |
GLP-1 lowers hepatic glucose output, which helps to lower blood sugars. It also stimulates the release of insulin by the pancreas. It also promotes satiety by affecting gut motility.
|
| Ghrelin | Mainly stomach, small amounts by small intestine and pancreas |
It stimulates appetite, increases food intake and promotes fat storage. Also known as the “hunger hormone”.
|
| Leptin | Produced in adipocytes of white adipose tissue | Leptin is a hormone produced by fat cells that helps regulate fat storage and balance energy levels. It signals to the brain when the body has enough energy stored, helping to control hunger and metabolism. |
| Peptide YY (PYY) | Small intestine (ileum) and the colon |
PYY main function is to reduce appetite and limit food intake. It deceases food intake by inhibiting gut motility.
|
Gastrointestinal Nutrient Absorption
It is important when seeing clients with various GI related diseases or concerns, to understand where nutrients are absorbed. This is particularly important when individuals have a compromised GI tract, such as absorption issues in relation to disease or surgery affecting a section of the GI tract. You can refer to the table below in practice.
| Part of the GI tract | Nutrients absorbed |
|---|---|
| Stomach |
|
| Duodenum |
|
| Jejunum |
|
| Ileum |
|
| Large Intestine |
|
Intestinal Transit Times
| Transit Stage | Transit Time (hrs) |
|---|---|
| 50% of stomach contents emptied | 2.5 – 3.0 |
| Total emptying of the stomach | 4.0 – 5.0 |
| 50% emptying of the small intestine | 2.5 – 3.0 |
| Transit through the colon | 30 – 40 |
There are gastrointestinal motility tests used to check transit times and for certain diseases. Examples of motility tests include:
- Gastrointestinal transit scintigraphy: uses a small amount of radioactive material to track food movement through the digestive tract.
- Radiopaque marker tests: involves swallowing markers visible on x-rays to measure food movement and colonic transit time.
- Hydrogen breath test: measures hydrogen in the breath to detect bacterial overgrowth or conditions like lactose intolerance.
- Esophageal manometry: measures pressure and contractions in the esophagus to diagnose swallowing problems.
You should explore what these tests (and others you may encounter) are traditionally used for and how they work.
GI Diseases
The following GI diseases will be covered in this section:
- Irritable Bowel Syndrome (IBS)
- Gastroesophageal Reflux Disease (GERD)
- Celiac Disease
- Diverticulitis
When you are evaluating the case study, you will notice it is focused on IBS and GERD, as this combination is common in practice. However, you will be provided with an overview and recommendations related to celiac disease and diverticulitis to use in practice.
Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is a common gastrointestinal (GI) disorder of gut-brain interaction characterized by recurrent abdominal pain associated with altered bowel habits.
The pathophysiology of IBS is not completely understood. There is a genetic predisposition and external triggers. Foods do not cause IBS, rather they do not allow tissues to heal by virtue of passing through. Pathological abnormalities include visceral hypersensitivity, abnormal motility disturbances, and psychological disturbances.
There are four “pillars” that are important to consider when evaluating IBS:
- Diet
- Psychological factors (e.g. anxiety, depression) and life stressors (e.g. divorce, family illness, financial difficulties, etc.)
- Exercise
- The microbiome (i.e. probiotic use).
The most common symptoms of IBS are chronic and recurring abdominal pain associated with abnormal bowel habits, categorized as:
- Diarrhea (loose and frequent stools, IBS-D)
- Constipation (hard and infrequent stools, IBS-C)
- Both diarrhea and constipation (IBS-M)
- Unspecified (IBS-U).
Some will only have excessive bloating or gas with normal BMs.
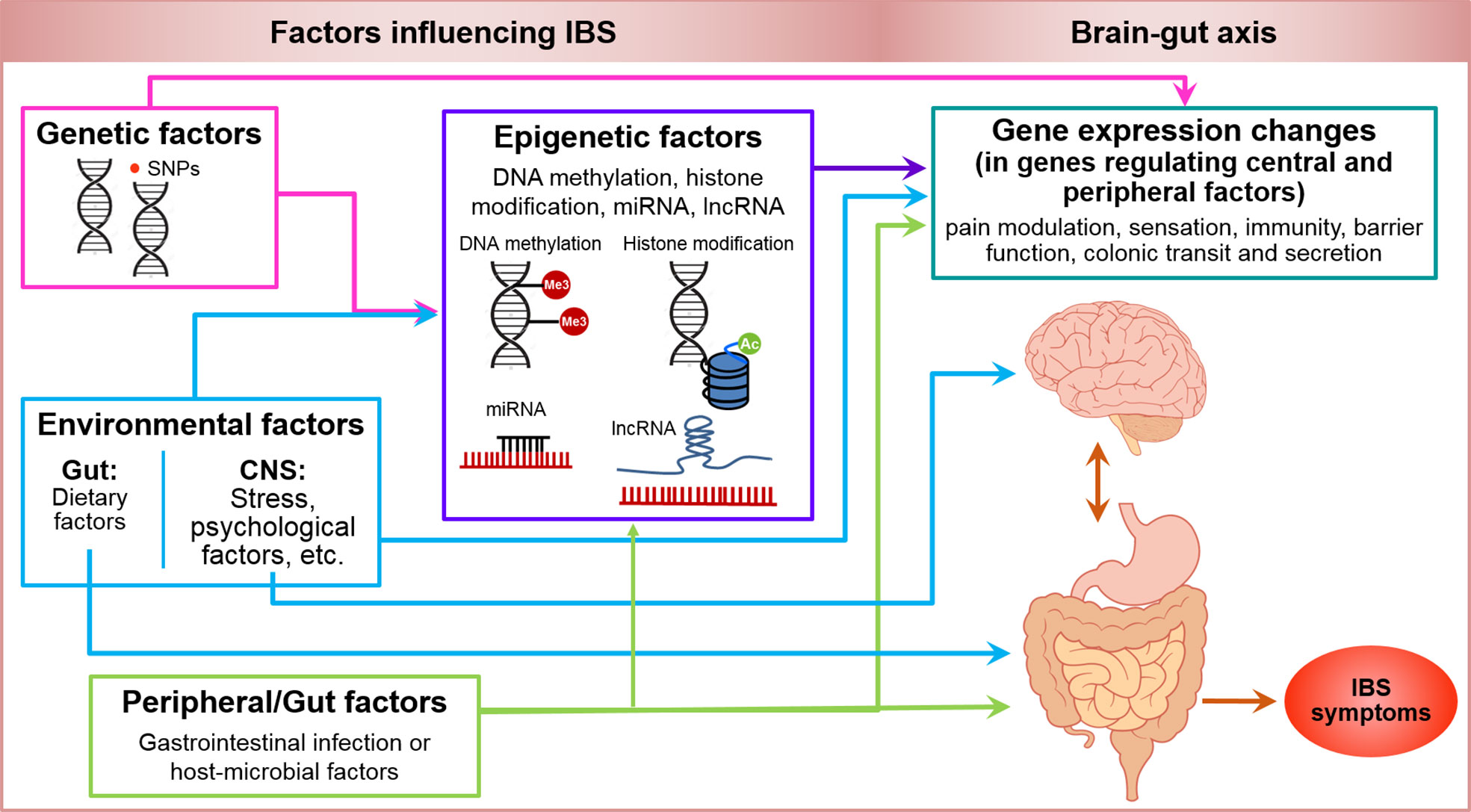
Source: Mahurkar-Joshi S and Chang L (2020) Epigenetic Mechanisms in Irritable Bowel Syndrome. Front. Psychiatry 11:805. doi: 10.3389/fpsyt.2020.00805
Screening and Diagnosis of IBS
Diagnosis of IBS can be difficult as there are no biochemical, structural or physiological abnormalities demonstrated in individuals with IBS. Thus, the diagnosis of IBS is symptom-based and not globally standardized. It remains a diagnosis of exclusion.
Clients may receive various testing (e.g. blood tests, CT scans, colonoscopy). However, a colonoscopy and blood test would typically be used to rule out IBS.
Once other GI conditions have been ruled out, physicians may use the Rome IV criteria. This is also a valuable tool for dietitians when evaluating a client’s symptoms. Under the Rome IV diagnostic criteria, a patient might have IBS if they experience one of the following:
- Recurrent abdominal pain symptoms
- Pain at least 1 day per week in the last 3 months, associated with 2 or more of the following:
- Pain related to defecation
- Change in the frequency of stool
- A change in the appearance of stool
However, the Rome criteria may not capture all IBS symptoms. For example, some individuals may experience severe bloating or gas without any noticeable changes in bowel movements. These symptoms can still be significant and often respond well to treatments like FODMAPs or probiotics. Therefore, it is important to use a variety of screening methods when assessing IBS to ensure the best care for your client.
Gastroesophageal Reflux Disease (GERD)
Reflux is a normal physiological function. Problems arise when there is an increased occurrence of transitory lower esophageal relaxation, leading to reflux of gas and liquids.
The involuntary backflow of gastric contents (acid, bile, pepsin, pancreatic enzymes) into the esophagus may result in reflux symptoms such as:
- Heartburn
- Regurgitation
- Chest pain
- Hiccups
- Nausea
- Bolus sensation
- Chronic cough
- Trouble swallowing
- Excessive mucus in the throat
Types of Reflux:
- Gastroesophageal Reflux Disease (GERD): when acid repeatedly refluxes from the stomach into the esophagus.
- Possible causes of GERD:
- Transient relaxation
- Decreased tone of lower esophageal sphincter (LES)
- Impaired clearance of esophagus
- Delayed gastric emptying
- Reduced salivation
- Possible causes of GERD:
- Laryngopharyngeal Reflux (LPR): when stomach acid travels up the esophagus and spills into the pharynx or larynx.
While treatment is similar for both conditions, it is important to distinguish between GERD and LPR, recognizing LPR symptoms is crucial for diagnosis.
Risk factors for reflux:
- Diet (high in refined carbohydrates)
- Increased gastric pressure
- Medical conditions (diabetes, eating disorders, hiatus hernia)
- Lifestyle (smoking)
- Medications
- Conditions affecting esophageal motility
Understanding these symptoms and risk factors is important when working with clients. However, a referral or previous GERD diagnosis often leads to a client seeking counselling from a dietitian.
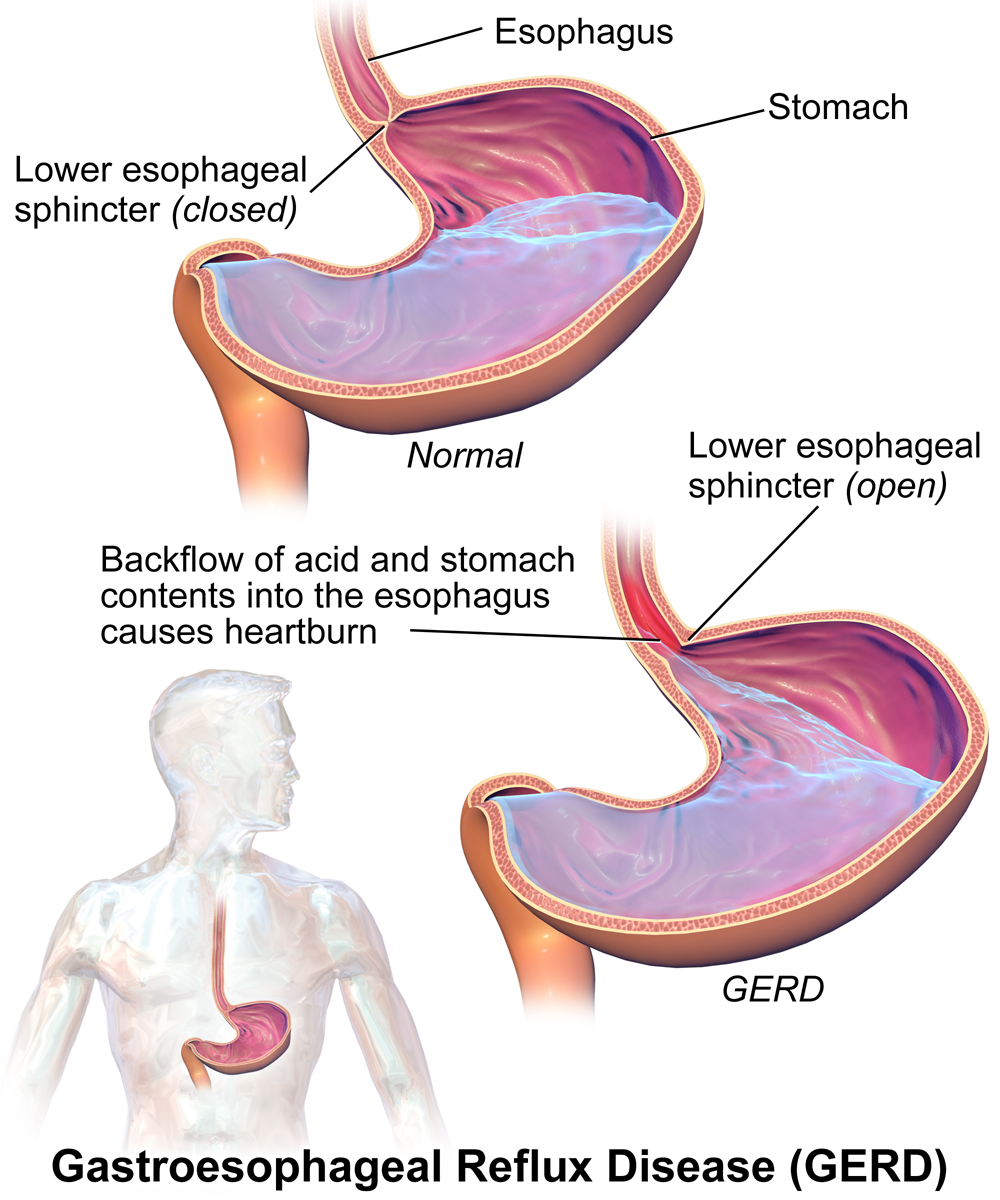
Source: BruceBlaus, CC BY-SA 4.0 , via Wikimedia Commons
Pharmacotherapy for Reflux
Those diagnosed with GERD are often on medications for reflux. The most common medications for reflux are Histamine 2 receptor blockers (H2 Receptor Blockers) and proton pump inhibitors (PPI’s). The table below describes these medications in more detail.
| Medication | Function | Common Examples |
|---|---|---|
| Proton Pump Inhibitors (PPIs) | Stop cells in the lining of the stomach from producing acid. They work by blocking a chemical system called the hydrogen-potassium adenosine triphosphatase enzyme system (otherwise known as the ‘proton pump’). |
|
| Histamine 2 Receptor Blockers | Block histamine-induced gastric acid secretion from the parietal cells of the gastric mucosa (lining of the stomach). |
|
Of the patients who use PPIs:
- 10% do not respond to them
- 40% will get only partial resolution of symptoms
- 15-20% will get significant side effects
- Short-term effects: nausea, gas, bloating, diarrhea, abdominal pain
- Long-term effects: increased risk of impaired nutrient absorption, ability to kill bacteria, C-difficile, pneumonia
An important consideration when a client is receiving these medications is to assess for nutrient deficiencies associated with long-term use (i.e. over a year of prolonged use). These include vitamin B12, iron, magnesium and calcium. As a dietitian, you should evaluate laboratory values for these nutrients of concern and potentially recommend dietary strategies or supplements, if necessary.
Recent studies regarding the long-term use of PPI medications have noted other potential adverse effects including: pneumonia, Clostridium difficile diarrhea, chronic kidney disease, risk of fractures, and dementia.
Celiac Disease
Celiac disease (CD) is an autoimmune disorder that permanently affects the digestive system. CD is triggered in genetically susceptible individuals by the ingestion of gluten.
There are three components to CD:
- The genetic factor is an individual’s genes and the possibility of CD
- The environmental factor is the exposure to gluten through diet
- The immunological component is the immune reaction that causes injury to the small intestine
When a person with CD consumes foods that contain gluten, it causes an immunological reaction in the small intestine, which damages the interior of the small intestine known as the villi. Villi line the interior of the small intestine and once they become inflamed and essentially flattened from the reaction with gluten, malabsorption of the nutrients needed for good health occurs.
It is important to explain to clients that ingestion of gluten will cause damage to the small intestine, even if they are not having symptoms. As a result, the only treatment is lifelong adherence to a gluten-free diet.
You should also explore non-celiac gluten sensitivity (NCGS) and how it differs from CD.
Screening and Diagnosis of CD
Diagnosis of CD can be difficult because of the broad range of symptoms that can vary from mild to severe. Although you may experience other screening tools, there are two common screening tools to be aware of:
- Serological Tests: and are recognized as the most sensitive and specific serological tests for CD diagnosis. To obtain accurate test results it is imperative that serological tests be performed prior to eliminating gluten from the diet. There is a potential for false positives (especially for those with other autoimmune disorders) or false negatives (ranging approximately from 10-15%, which may be related to an IgA deficiency). You should explore and develop a further understanding of these tests. Positive serology in insolation is insufficient for diagnosis of celiac disease.
- Small Intestinal biopsy: Currently the only definitive test and the gold standard for diagnosing CD is the small intestinal biopsy where cells or tissues are removed for examination of villous atrophy (damage of the villi in the small intestine). It is important that biopsies be taken prior to eliminating gluten from the diet as histological findings can begin to normalize upon initiation of a gluten free diet.
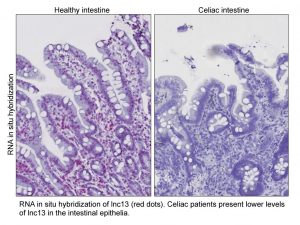
Source: Photo by Invited Researcher is licensed under CC BY-NC-ND 4.0
It is important to understand that we are learning so much about celiac disease as the symptoms vary tremendously. For example, someone with severe symptoms could have a low IgA-tTG, while someone with a high IgA-tTG may have next to no symptoms and have trouble understanding their diagnosis. Based on clinical experience and expertise, you may notice that people will refuse testing because they have already started following a gluten-free diet, have had a relief of symptoms, and it is very painful to go back to eating gluten for the purposes of testing.
Diverticulitis
Diverticula are small, bulging pouches that can form in the lining of the large intestine (most often in the lower part).
Diverticulosis is the presence of diverticula, and it is quite common as people age. According to figures from the United States National Institute of Health, diverticulosis is experienced by more than 30% of adults aged 50-59 and more than 70% of adults aged 80 and older.
The brief video below (1:24) demonstrates diverticulosis during a colonoscopy.
https://www.youtube.com/watch?v=ThuBZxbLo9g
Diverticulitis is a condition that occurs when one or more of the diverticula pouches become inflamed, and in some cases infected. Diverticulitis can cause severe abdominal pain, fever, nausea and a marked change in bowel habits.
- Risk Factors: aging, obesity, smoking, lack of exercise, diet high in animal fat or low in fiber
- Symptoms: pain, nausea, vomiting, fever, constipation, blood or mucus in stool
- Treatments: often focused on prevention (e.g. high fiber diet, fiber supplements, medicines, probiotics)
- Diagnosis: CT scan or colonoscopy
Management of diverticulitis is controversial as a result of limited evidence. This will be explained in more detail during the “Plan” section.
The stage in which the stomach responds to the mere sight, smell, taste, or thought of food.
The stage in which swallowed food and semi-digested proteins stimulate gastric activity.
The stage in which the duodenum responds to chyme and moderates gastric activity through hormones and nervous reflexes.
hydrochloric acid
Immunoglobulin A Tissue Transglutaminase
Immunoglobulin A Endomycial Antibodies

